You’ve just ordered new busbars for your switchgear panel. The supplier offers three options: bare copper (the cheapest), tin-plated (mid-range), or silver-plated (premium). All carry the same rated current. All meet IEC standards. So why would you ever pay more?
Three months after installation, you get a call: a connection joint is running hot. The infrared camera shows 15°C above design limits. Root cause? That “bargain” bare copper busbar has begun oxidizing, and the oxide layer—a poor conductor—has pushed contact resistance through the roof. Now you’re facing emergency maintenance, potential equipment damage, and the uncomfortable truth: the cheapest busbar often costs the most over its lifetime.
Why Busbar Coating Matters: The Hidden Enemy Is Oxidation
Copper is one of the best electrical conductors on Earth—but only when it’s clean and pure. The moment it touches air, chemistry takes over.
Bare copper oxidizes readily, forming copper oxide (CuO) or more complex compounds like copper carbonate. These oxides are semi-insulators, not conductors. Even a thin 1–2 micrometer layer can increase contact resistance measurably. As oxidation deepens, resistance grows exponentially. This isn’t a cosmetic issue; it’s a failure mechanism.
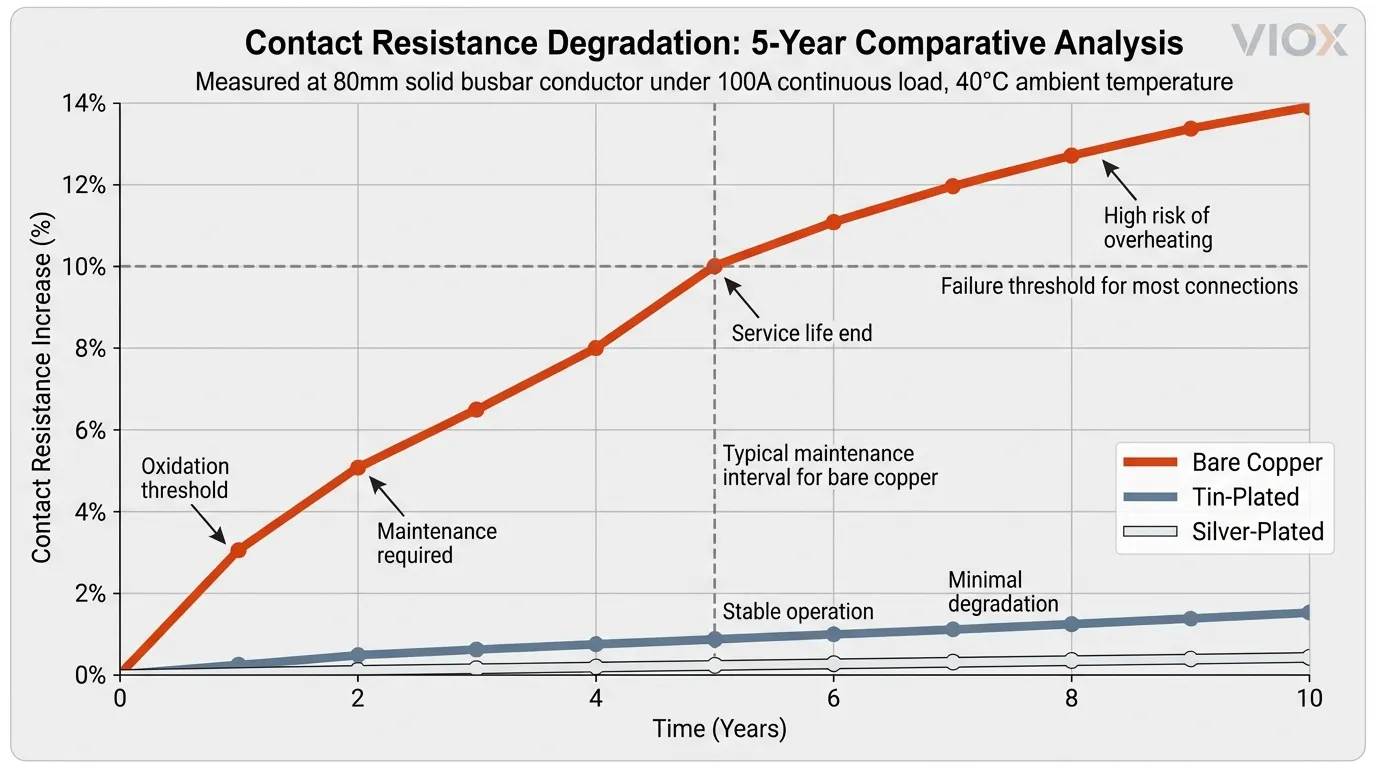
The consequence is a vicious cycle:
- Oxidation raises contact resistance (R)
- Higher resistance generates heat under load (P = I²R)
- Heat accelerates further oxidation
- Connections eventually fail due to overheating or embrittlement
This is why the electrical industry doesn’t leave this to chance. IEC 60947-2 (the standard governing industrial switchgear) recognizes that surface condition directly impacts reliability. The question isn’t whether to coat your busbar—it’s which coating to choose.
Deep Dive: Bare Copper

Initial appeal: Bare copper exhibits the highest theoretical conductivity (58 MS/m, approximately 100% IACS). If you’re building a short-term, low-criticality circuit in a dry, temperature-controlled laboratory, bare copper works.
The reality:
- Salt spray testing (ASTM B117): Bare copper survives ~120 hours before visible corrosion becomes problematic
- Contact resistance: Baseline at 16 µΩ for an 80mm solid bar, but increases 8–12% within 5 years in typical indoor humidity
- Maintenance burden: Requires periodic cleaning, re-torquing, and application of conductive grease (like Penetrox or Noalox) to prevent oxidation
Best for:
- Temporary installations or test circuits
- Strictly climate-controlled dry environments (museums, sealed server rooms below 30% relative humidity)
- Budget-conscious applications with planned replacement cycles (<3 years)
Not recommended for: Marine environments, industrial sites, outdoor installations, or any long-term reliability requirement.
Deep Dive: Tin-Plated Copper

Why tin works: Tin is less reactive than copper. While tin does oxidize (forming tin oxide), the oxide layer is extremely dense and adheres tightly to the base metal, effectively sealing the underlying copper from further environmental attack.
The data:
- Salt spray testing: Tin-plated busbars typically withstand 720+ hours (6× longer than bare copper)
- Contact resistance stability: <2% increase over 5 years in humid environments
- Plating thickness: Industry standard is 5–15 µm; some applications use up to 50 µm in extreme environments
- Conductivity trade-off: Tin is ~5× less conductive than copper, but the plating thickness is so small (nanoscale relative to busbar dimensions) that it contributes negligibly to overall resistance
Galvanic advantage: When tin-plated copper contacts aluminum (common in battery systems, solar inverters), the tin acts as an intermediary metal, reducing the electrochemical potential difference from ~2.0V (bare copper-aluminum) to manageable levels. This prevents accelerated galvanic corrosion of aluminum.
Best for:
- Industrial switchgear and distribution boards
- Renewable energy systems (solar, wind, storage)
- Data centers and critical infrastructure
- Environments with humidity, salt spray, or chemical fumes
- Aluminum-copper mixed assemblies
Deep Dive: Silver-Plated Copper
Why silver is premium: Silver has the highest electrical conductivity of any metal (64 MS/m) and remains conductive even when tarnished. Silver sulfide (the tarnish that forms in sulfur-rich air) is still a reasonably good conductor, unlike copper oxide.
The data:
- Contact resistance: Lowest among all options; enables higher temperature rise limits (IEC 60947-2 permits 70K for low-voltage silver-plated contacts vs. 60K for bare copper)
- Longevity: Minimal degradation even in sulfur-rich industrial environments
- Plating thickness: Typically 5–20 µm, with specialized high-wear applications using up to 25 µm
- Cost impact: 2–3× the cost of tin-plated busbar
When silver outperforms tin: In high-voltage switchgear (IEC 62271-1 standard for medium and high-voltage), silver-plated sliding contacts are mandatory for low-temperature-rise performance. For deeper insight into how this relates to contact materials and arc suppression mechanisms, see our guide to AC contactor components and design logic. High-current breakers and switch contacts operating at 110kV+ rely on silver.
Trade-offs:
- Silver is soft; repeated mechanical rubbing (sliding contacts) can wear plating faster than tin
- Silver requires compatible grease in high-vibration environments to prevent “galling” (adhesive wear)
Best for:
- High-current joints requiring minimal temperature rise (HV breakers, large busbars >500A)
- Sliding or cyclic contact applications
- Military and aerospace where cost is secondary to reliability
- Environments with elevated sulfur content where copper oxide would degrade quickly
Comparison Table: Quick Selection Matrix
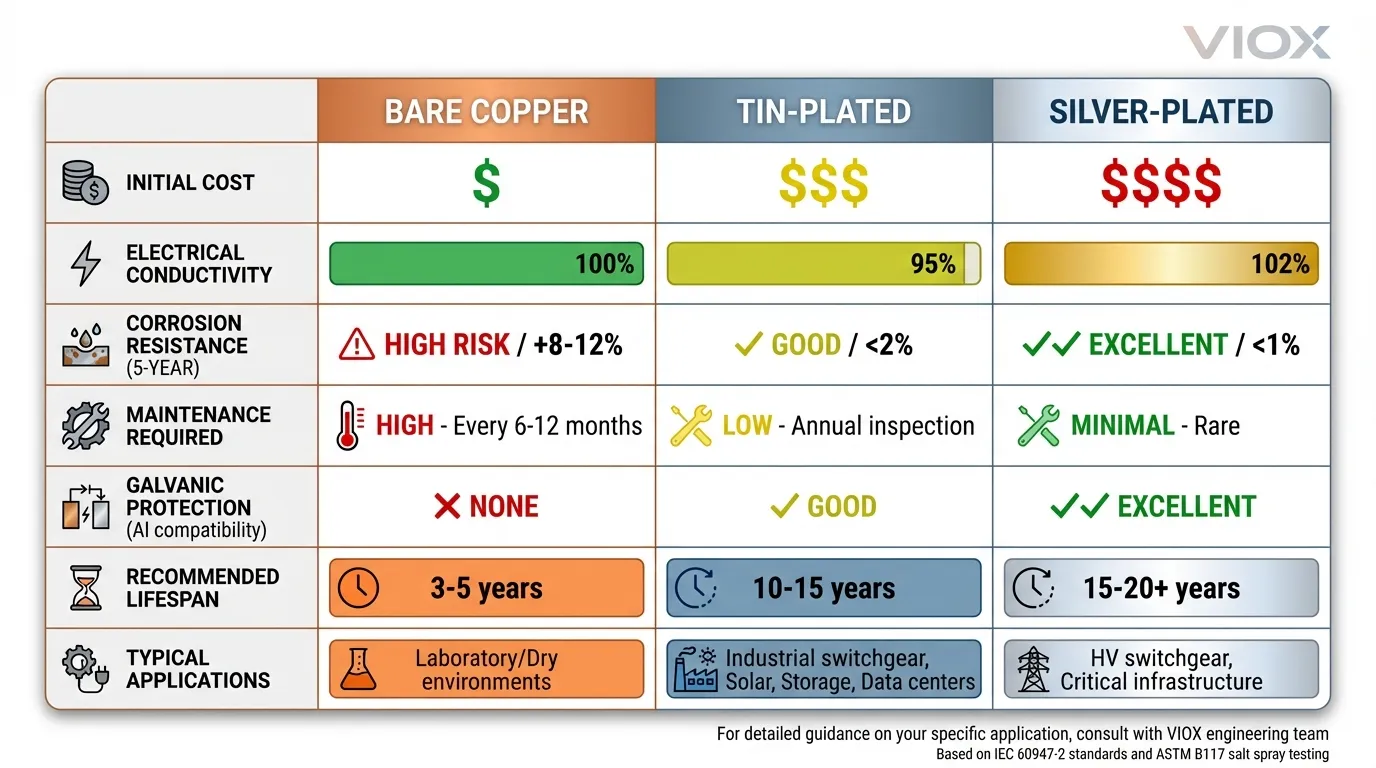
| Feature | Bare Copper | Tin-Plated | Silver-Plated |
|---|---|---|---|
| Initial Cost | $$ | $$$ | $$$$ |
| Electrical Conductivity | 100% | ~95% (effective) | 102% |
| Contact Resistance Stability (5-year) | +8–12% | <2% | <1% |
| Salt Spray Rating (ASTM B117) | 120 hrs | 720+ hrs | 1000+ hrs |
| Maintenance Required | High (6–12 months) | Low (annual inspection) | Minimal |
| Galvanic Protection (with Al) | None | Good | Excellent |
| Recommended Lifespan | 3–5 years | 10–15 years | 15–20+ years |
| Typical Applications | Lab/dry environments | Industrial switchgear, solar, storage | HV switchgear, critical infrastructure |
Real-World Impact: Galvanic Corrosion & Aluminum Compatibility
In modern electrical systems—especially solar arrays and battery storage—you often encounter aluminum conductors or lugs connected to copper busbars. This junction represents a classic galvanic cell scenario, and proper surface coating is the proven engineering solution to ensure reliable electrical connections that will last the system’s designed lifetime.
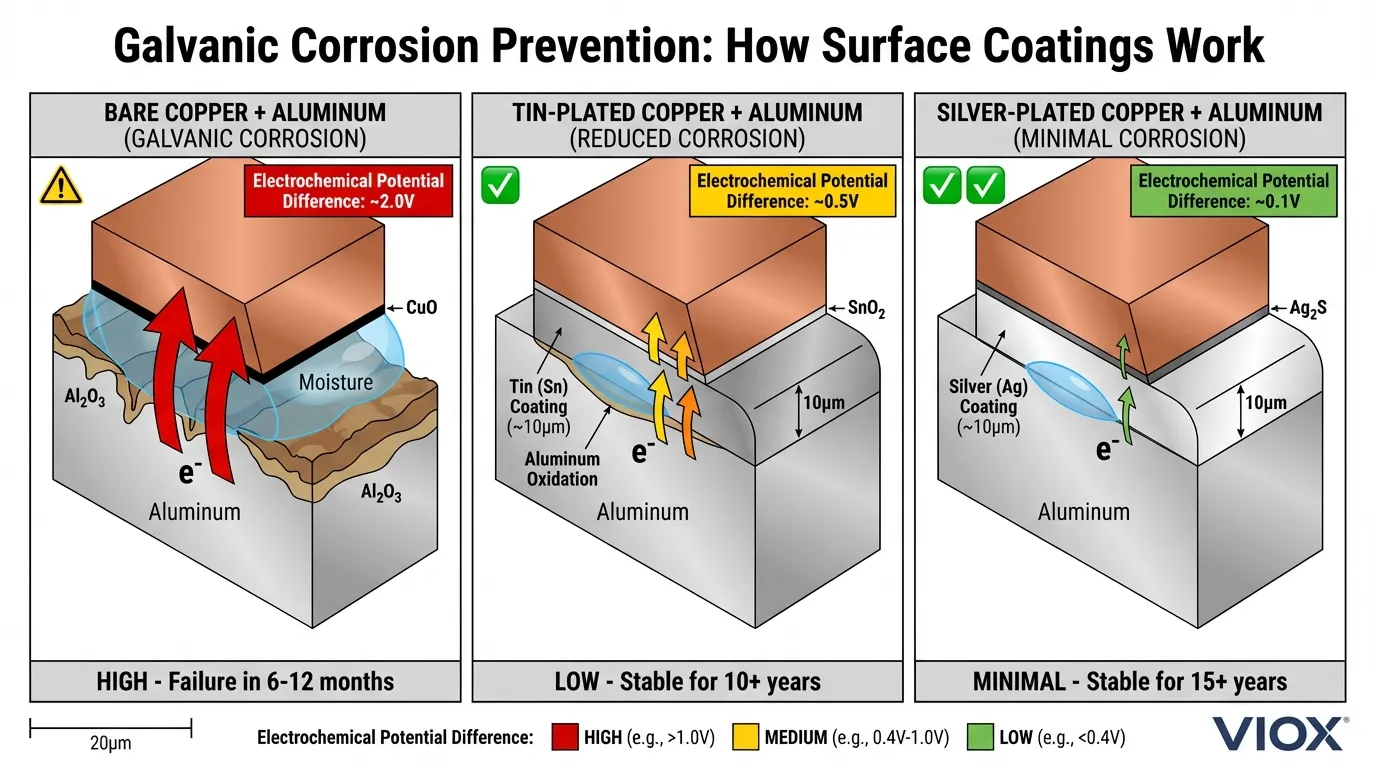
When bare copper and aluminum meet in the presence of moisture:
- Electrochemical potential difference: ~2.0V
- Aluminum (more reactive) sacrifices electrons
- Aluminum oxidizes to Al₂O₃, a hard, non-conductive layer
- Contact resistance skyrockets; connection fails
With tin-plated copper: The tin layer reduces the potential difference, slowing galvanic corrosion substantially. Combined with proper jointing compound (zinc-suspended grease), the joint remains stable for 10+ years.
With silver-plated copper: The potential difference is minimized even further, providing superior long-term protection.
Application Scenarios
Scenario 1: Residential 230V Distribution Panel
Load: 100A residential feeder with resistive loads (heating, lighting)
Environment: Dry indoor mounting
Recommendation: Bare copper acceptable if panel will be upgraded within 5 years; tin-plated preferred for 10-year reliability at modest cost premium.
Scenario 2: Solar PV Combiner Box (600V DC)
Load: 60A DC from parallel strings to inverter input
Environment: Outdoor, high humidity, temperature cycling
Complication: Aluminum terminal lugs on DC combiner side
Recommendation: Tin-plated copper mandatory to prevent galvanic corrosion at aluminum junction.
Scenario 3: Data Center Power Distribution
Load: 400A three-phase feeders
Environment: Climate-controlled, but continuous operation
Recommendation: Tin-plated copper standard. Silver-plated only if temperature rise becomes a bottleneck (rare unless undersizing components).
Scenario 4: High-Voltage Breaker Assembly (110kV Class)
Load: 1200A main contacts
Environment: Outdoor pole-mounted or indoor switchyard
Recommendation: Silver-plated sliding contacts mandatory per IEC 62271-1. Tin-plated not acceptable for this duty. For reference on how utilization categories relate to electrical load switching and busbar selection, review our guide to IEC 60947-3 utilization categories.
FAQ: Your Busbar Coating Questions Answered
Q1: Can I clean oxidized bare copper and avoid plating?
A: Temporarily, yes. Wire-brushing followed by conductive grease (Penetrox, Noalox) removes oxidation and improves contact resistance. However, the oxide will return within months in humid environments. For temporary fixes, this works; for permanent solutions, plating is more reliable.
Q2: Does tin plating affect the breaker’s breaking capacity (Icu)?
A: No. Breaking capacity is determined by arc-quenching design, not surface coating. However, lower contact resistance (improved by plating) does reduce temperature rise, potentially allowing higher continuous current capacity indirectly. See our MCCB selection guide for details.
Q3: Is there any environment where silver-plating degrades faster than tin?
A: Yes—high-sulfur industrial areas. Silver forms sulfide tarnish (which is still conductive but less desirable aesthetically). Tin remains unchanged. If appearance or sulfur-resistance is critical, tin is actually superior in that specific scenario.
Q4: Can I mix bare copper and tin-plated busbars in the same panel?
A: Electrically, yes—if they’re not directly connected. However, it’s poor practice because maintenance becomes complex: one part needs cleaning/greasing every 6 months, the other doesn’t. Standardize on one coating per panel.
Q5: How do I inspect a busbar to detect oxidation before failure?
A: Thermal imaging is the gold standard. A corroded joint will show 10–20°C higher surface temperature under rated load. Visual inspection works too: greenish tint on copper = active corrosion; dull gray/silver on tin-plated or silver-plated = normal patina (not problematic). Annual thermographic scanning during peak load is recommended for critical panels. For best practices on maintaining electrical equipment, consult our industrial maintenance and inspection checklist.
Q6: What’s the environmental cost of tin or silver plating?
A: Plating processes generate wastewater requiring treatment, but the extended lifespan (10–20 years vs. 3–5 years for bare copper) reduces total lifecycle material waste. Over 20 years, tin-plated busbars typically generate 40–50% less waste than repeated bare copper replacement. From a sustainability perspective, coating busbars is the right choice for long-term installations.
Key Takeaways
- Bare copper starts at 100% conductivity but degrades rapidly under humidity; useful only for dry, short-term applications or budget-conscious temporary setups.
- Tin-plated copper is the industry standard for industrial switchgear, renewable energy, and aluminum-compatible assemblies; offers 10–15 year lifespan with minimal maintenance at a modest cost premium.
- Silver-plated copper is reserved for high-current, high-reliability applications where temperature rise must be minimized (HV switchgear, data center distribution) or where sliding contacts require superior wear resistance.
- Galvanic corrosion is real: Never connect bare copper to aluminum without coatings or protective grease. Tin or silver plating is the proper engineering solution.
- Cost is not the limiting factor: A $50–100 premium for tin plating is recouped within the first 2–3 years through avoided maintenance and prevented failures.
- IEC 60947-2 permits higher temperature rise for plated contacts, potentially enabling slightly higher current capacity indirectly—another hidden benefit of coating investment.
Choose Reliability. Choose VIOX.
At VIOX Electric, we manufacture busbars engineered to IEC 60947-2 standards with certified plating processes and rigorous quality control. Whether you need bare copper for testing, tin-plated for industrial reliability, or silver-plated for critical infrastructure, VIOX delivers the coating you specify—backed by technical expertise and decades of industry trust.
Questions about busbar coating selection for your specific application? Our engineering team is ready to help. Contact VIOX today for a consultation.


